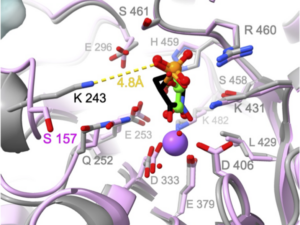
A team of researchers, including Jillian Milanes, Samuel Kwain, Dan Whitehead, and Jim Morris, have published a groundbreaking study in PLOS Pathogens titled “Enolase inhibitors as therapeutic leads for Naegleria fowleri infection.” The study explores the potential of enolase inhibitors, originally developed for treating glioblastoma, as a new approach to combat the life-threatening brain infection caused by the amoeba Naegleria fowleri. Their findings suggest that the inhibitor HEX shows promise in extending survival in animal models, marking a significant step forward in the search for effective treatments against this often fatal condition. Read the article.
864-656-EPIC
clemson_epic@clemson.edu
