Catalyzed Enhanced Reactivity of Amines Through Weak Noncovalent Interactions
Andrew Storer (1), Amaechi Odoh (1), Austin Seilkop (1) and Byoungmoo Kim
(1) Department of Chemistry
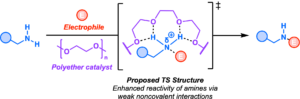
Polyamino systems are common in bioactive molecules, and selective modification of these motifs would provide a new avenue for drug discovery.1 Yet, catalytic site-selective functionalization of these polyamines remains underexplored due to the high the lack of catalytic methods and the inherent reactivity of amines.2,3 The current state-of-the-art catalytic methods utilize nucleophilic catalysts, enzymes, or metal-catalyzed systems to undergo desymmetrization of meso and prochiral diamines.3–9 However, there is a limited scope of N-functionalization. There has yet to be a direct catalytic approach that enables diversification of amines. To address this challenge, we propose an alternative strategy to develop a new method that enhance the nucleophilicity of amine towards various electrophiles. This so-called “activation” strategy has been highly explored in the literature for O-functionalization of alcohols, but not with amines. Herein, we report a new strategy and catalyst design for the activation of amines using polyether catalysts as a H-bond acceptor. This poster will show our catalyst design for the rate acceleration of amines by using a simple N-arylation reaction as a model system. We observed optimal rate acceleration when we designed our catalyst to be a linear chain with flexible structural architecture. Based on these findings, we are currently developing a new chiral polyether catalyst for kinetic resolution of racemic amines.
References
(1) Ertl, P.; Altmann, E.; Mckenna, J. M. The Most Common Functional Groups in Bioactive Molecules and How Their Popularity Has Evolved over Time. J. Med. Chem. 2020, 63 (15), 8408–8418. https://doi.org/10.1021/acs.jmedchem.0c00754.
(2) Pellissier, H. Catalytic Non-Enzymatic Kinetic Resolution. Adv. Synth. Catal. 2011, 353 (10), 1613–1666. https://doi.org/10.1002/adsc.201100111.
(3) De, C. K.; Seidel, D. Catalytic Enantioselective Desymmetrization of Meso -Diamines: A Dual Small-Molecule Catalysis Approach. J. Am. Chem. Soc. 2011, 133 (37), 14538–14541. https://doi.org/10.1021/ja2060462.
(4) Bustos, E.; Gotor-Fernández, V.; Montejo-Bernardo, J.; García-Granda, S.; Gotor, V. First Desymmetrization of 1,3-Propanediamine Derivatives in Organic Solvent. Development of a New Route for the Preparation of Optically Active Amines. Org. Lett. 2007, 9 (21), 4203–4206. https://doi.org/10.1021/ol701720m.
(5) Ríos-Lombardía, N.; Busto, E.; García-Urdiales, E.; Gotor-Fernández, V.; Gotor, V. Enzymatic Desymmetrization of Prochiral 2-Substituted-1,3-Diamines: Preparation of Valuable Nitrogenated Compounds. J. Org. Chem. 2009, 74 (6), 2571–2574. https://doi.org/10.1021/jo8025912.
(6) Ríos-Lombardía, N.; Busto, E.; Gotor-Fernández, V.; Gotor, V. Chemoenzymatic Asymmetric Synthesis of Optically Active Pentane-1,5-Diamine Fragments by Means of Lipase-Catalyzed Desymmetrization Transformations. J. Org. Chem. 2011, 76 (14), 5709–5718. https://doi.org/10.1021/jo2007972.
(7) Kitagawa, O.; Matsuo, S.; Yotsumoto, K.; Taguchi, T. Catalytic Asymmetric Desymmetrization of Meso-Diamide Derivatives through Enantioselective N-Allylation with a Chiral π-Allyl Pd Catalyst: Improvement and Reversal of the Enantioselectivity. J. Org. Chem. 2006, 71 (6), 2524–2527. https://doi.org/10.1021/jo052488y.
(8) Anstiss, M.; Nelson, A. Catalytic and Stoichiometric Approaches to the Desymmetrisation of Centrosymmetric Piperazines by Enantioselective Acylation: A Total Synthesis of Dragmacidin A. Org. Biomol. Chem. 2006, 4 (22), 4135–4143. https://doi.org/10.1039/b608910k.
(9) Kitagawa, O.; Yotsumoto, K.; Kohriyama, M.; Dobashi, Y.; Taguchi, T. Catalytic Asymmetric Synthesis of Vicinal Diamine Derivatives through Enantioselective N-Allylation Using Chiral π-Allyl Pd-Catalyst. Org. Lett. 2004, 6 (20), 3605–3607. https://doi.org/10.1021/ol048498n.
 University Home
University Home Chemistry
Chemistry Apply!
Apply! Give
Give