Catalytic substrate-selective silylation and uranium binding studies using bifunctional macrocycles
Austin Seilkop, Kayleigh Wahr and Boni Kim
Department of Chemistry
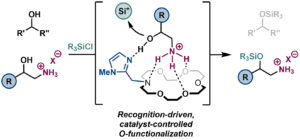
In recent years, synthetic chemists have sought to create artificial catalysts that mimic enzymes, which can perform substrate-selective reactions in complex mixtures using multiple non-covalent interactions. However, substrate-selective catalysis remains less explored compared to chemo-, regio-, and enantio-selective catalysis. Taking this into consideration, our group aims to further develop the field of substrate-selective catalysis by designing catalytic methods that enable protecting group-free hydroxyl modification of amino alcohols. Performing selective amino alcohol functionalization faces two main limitations: the need for multi-step protecting group methods to achieve specific reactions, and the impediment of transition-metal catalysts due to strong binding with hydroxyl and amino groups. To address this challenge, we devised an alternative strategy by leveraging ammonium-recognition of crown ethers and multiple weak non-covalent interactions to enable substrate-selective functionalization of amino alcohols. Herein, we have developed a novel, bifunctional crown ether-based organocatalyst for the silylation of hydroxyl groups via ammonium-binding recognition-driven selectivity. Mechanistic studies based on substrate competition experiments show catalyst-controlled silylation of ammonium-alcohols over aliphatic alcohols, including natural products, up to >20:1 selectivity. In the future, we hope to use this strategy for peptides and complex aminoglycoside antibiotics. We can also extend the application of these macrocyclic compounds to bind radioactive metals such as uranium. By varying the electronic effects and pocket size on the macrocyclic ligands, we can further study the change in redox potential of uranium and the stability of the actinide complexes, respectively.
 University Home
University Home Chemistry
Chemistry Apply!
Apply! Give
Give