Titanium Catalyzed Multi-functionalization of Carboxylic acids for The Synthesis of Chiral Amines
Samirah Muhammad (1), Giovani Gutierrez (1), Jason A. Wilt (1) and Dr. Byoungmoo Kim (1)
(1) Department of Chemistry
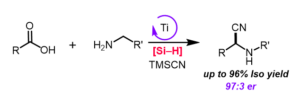
Chiral amines are important motifs that are present in many bioactive molecules, natural products, and pharmaceuticals. Therefore, there has been a continuous demand for more efficient enantioselective methods to synthesize chiral amines. The Strecker reaction is one of the well-established ways for the enantioselective synthesis of chiral amines, which involves carbonyl, an amine, and cyanide starting materials. However, the carbonyl starting materials are often unstable which limits the utility of this reaction. On the other hand, the corresponding carboxylic acids are desirable candidates to use since they are bench stable, non-toxic, inexpensive, and abundant building blocks. Despite these advantages, there are only a few examples of the reductive Strecker reaction using carboxylic acids and there are no known enantioselective variants.
Additionally, current examples of the reductive Strecker reaction often use precious metal catalysts such as iridium, which can be expensive and impact scalability. To address these challenges, we describe our efforts in the Kim Group employing a first-row transition metal, specifically titanium, as a catalyst, which is inexpensive, non-toxic and offers diverse reactivities. Our method uses a single titanium catalyst to take various carboxylic acids to enantio-enriched amine products in one-pot without the need for multiple catalysts or intermediate purification making this method more efficient. Our current focus is to demonstrate a gram scale synthesis of the method and expand the multi-catalytic strategy to the catalytic decomposition of plastic wastes.
 University Home
University Home Chemistry
Chemistry Apply!
Apply! Give
Give