Determining protonation site in fentanyl protomers using ion mobility-aligned MS/MS fragmentation
Ralph Aderorho and Christopher D. Chouinard
Publication Date: December 14, 2023
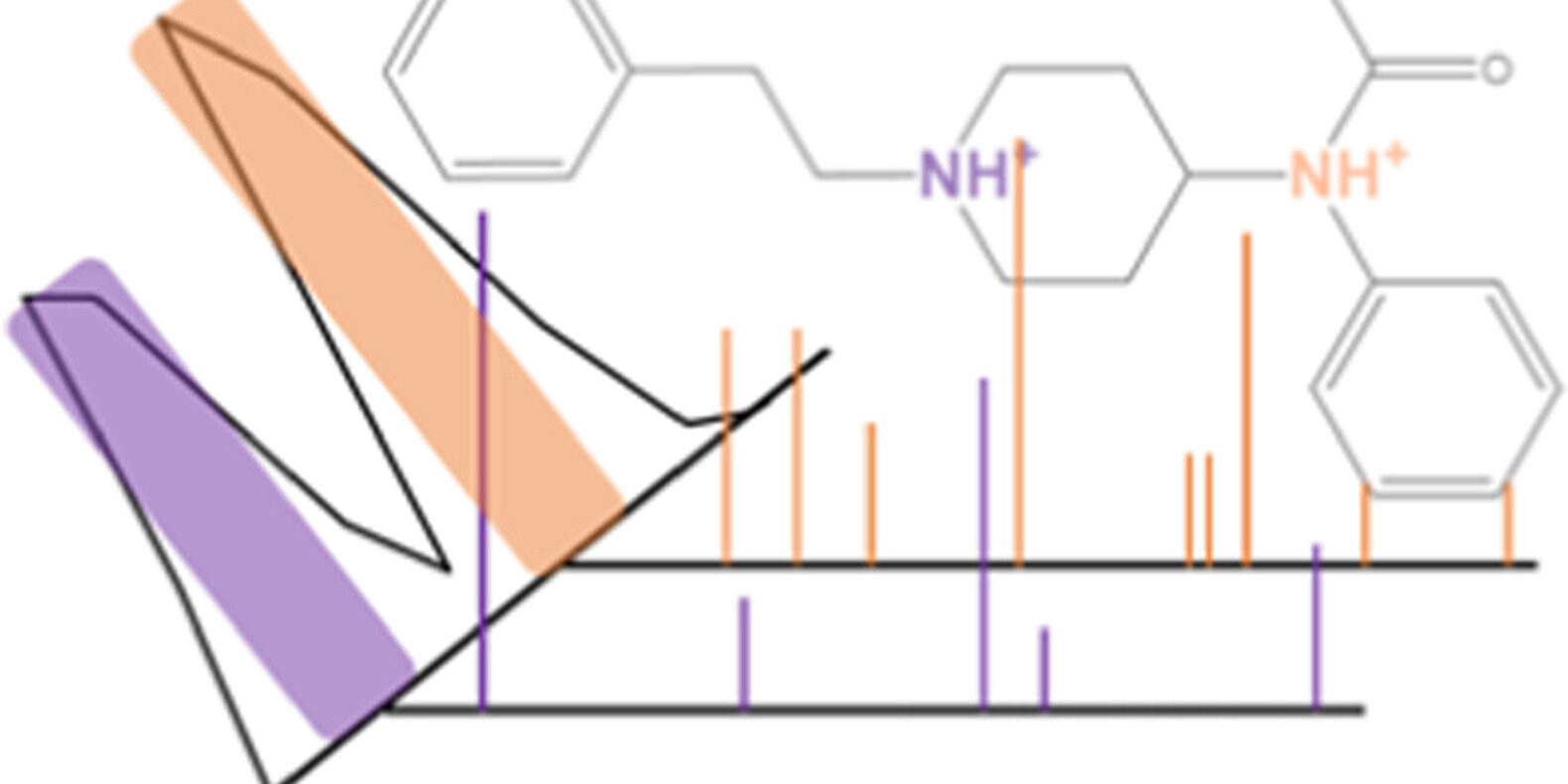
Drug overdoses in the United States totalled over 100,000 in 2022, with an estimated 68 % of those being attributed to illicitly manufactured fentanyls (IMFs). Their ease and low cost of synthesis have prompted a proliferation of new fentanyl analogues in the illicit drug market, and further created demand for analytical tools capable of identifying these new substances. Ion mobility-mass spectrometry (IM-MS) has emerged as a strong candidate to lead this effort, but the presence of multiple mobility peaks for a single fentanyl compound has created ambiguity in analysis. Herein, we use a combination of IM and tandem mass spectrometry (MS/MS) and determine that the multiple mobility peaks correspond to protonation site isomers, or ‘protomers’, where protonation can occur at either the N-piperidine ring and N-amide. Unique fragmentation spectra and energy-resolved CID revealed characteristic dissociation pathways for each protomer. Furthermore, this approach was applied to another analogue, despropionyl ortho-methylfentanyl, and was subsequently able to annotate its protomers as well. This approach holds tremendous promise for identification of future novel opioids including fentanyl analogues.