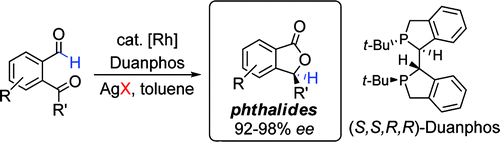
2. Phthalides by Rhodium-Catalyzed Ketone Hydroacylation
Phthalides are biologically relevant five-membered lactones found in herbs, fruits, and vegetables. Herein we communicate the first atom-economical approach to phthalides by using enantioselective ketone hydroacylation. In the presence of Rh[(Duanphos)]X (X = NO3, OTf, OMs), various 2-ketobenzaldehydes undergo intramolecular hydroacylation to produce phthalide products in good yields and 92−98% ee’s. Our study highlights the key role counterions play in controlling both reactivity and enantioselectivity. A concise asymmetric total synthesis of the celery extract (S)-(−)-3-n-butylphthalide is also presented.
Download PDF »
View on PubMed »
Phan, D. H. T.; Kim, B.; Dong, V. M. J. Am. Soc. Chem. 2009, 131, 15608–15609.