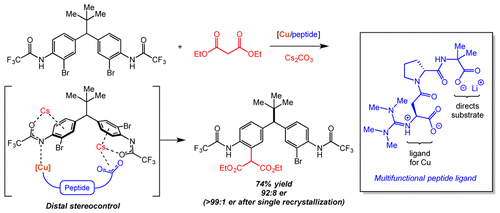
4. Distal Stereocontrol Using Guanidinylated Peptides as Multifunctional Ligands: Desymmetrization of Diarylmethanes via Ullmann Cross-Coupling
We report the development of a new class of guanidine-containing peptides as multifunctional ligands for transition-metal catalysis and its application in the remote desymmetrization of diarylmethanes via copper-catalyzed Ullman cross-coupling. Through design of these peptides, high levels of enantioinduction and good isolated yields were achieved in the long-range asymmetric cross-coupling (up to 93:7 er and 76% yield) between aryl bromides and malonates. Our mechanistic studies suggest that distal stereocontrol is achieved through a Cs-bridged interaction between the Lewis-basic C-terminal carboxylate of the peptides with the distal arene of the substrate.
Download PDF »
View on PubMed »
Kim, B.; Chinn, A. J.; Fandrick, D. R.; Senanayake, C. H.; Singer, R. A.; Miller, S. J. J. Am. Chem. Soc. 2016, 138, 7939–7945.