Publications
Since Clemson
(†undergraduate student)
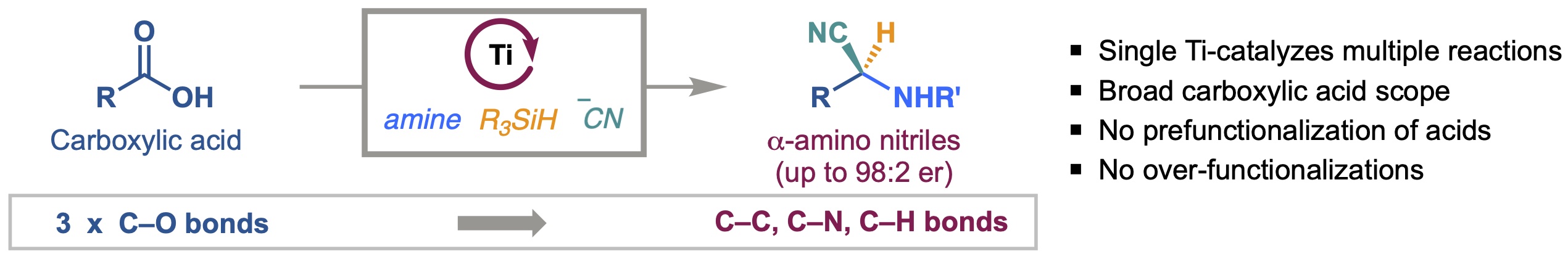
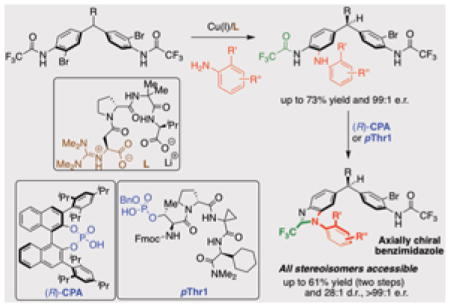
14. Enantioselective Deoxygenative Amino-Cyanation of Carboxylic Acids via Ti-Multicatalysis
Gutierrez, G.; Wilt, A. J.; Muhammad, S.; Girotti, E.†; Rodriguez, D.†; Kim, B.*
Org. Lett. 2024, 26 (44), 9442–9447
• One of the Most Read Articles in Org. Lett. in Nov.
• Highlighted in OPRD newsletter “Some Items of Interest to Process R&D Chemists and Engineers”
• Highlighted in Synfacts 2025; 21(01): 53.
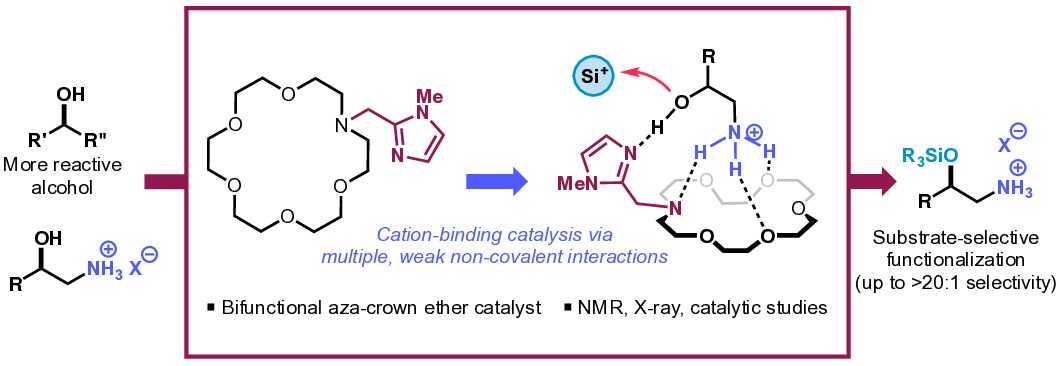
13. Ammonium-Binding Bifunctional Aza-Crown Ether Catalysts for Substrate-Selective Hydroxyl Functionalization
Seilkop, A. G.; Odoh, A. S.; Coradi, N. J.†; Wright, J. I.†; Barroso, J.; Kim, B.*
J. Org. Chem. 2024, 89 (18), 13338–13344
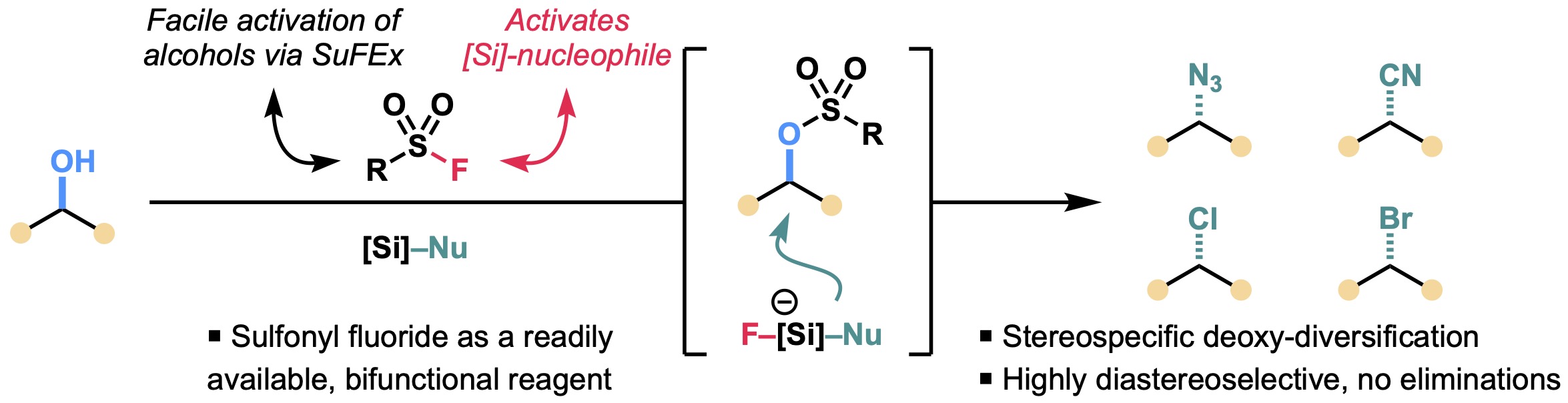
12. SuFEx-Enabled Direct Deoxy-Diversification of Alcohols
Odoh, A. S.; Keeler, C.†; Kim, B.*
Org. Lett. 2024, 26 (18), 4013–4017
• One of the Most Read Articles in Org. Lett. in May and June 2024.
• Highlighted in OPRD newsletter “Some Items of Interest to Process R&D Chemists and Engineers”
11. Asymmetric Catalysis Mediated by Synthetic Peptides, Version 2.0: Expansion of Scope and Mechanisms
Metrano, A. J.; Chinn, A. J.; Shugrue, C. R.; Stone, E. A.; Kim, B.; Miller, S. J.
Chem. Rev. 2020, 120, 11479–11615.
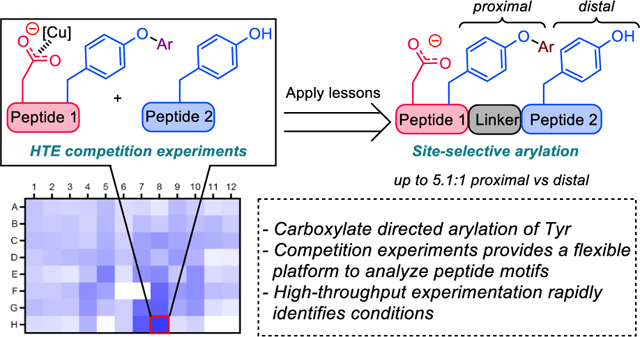
10. Application of High-Throughput Competition Experiments in the Development of Aspartate Directed Site-Selective Modification of Tyrosine Residues in Peptides
Chinn, A. J.; Hwang, J.; Kim, B.; Parish, C. A.; Krska, S. W.; Miller, S. J.
J. Org. Chem. 2020, 85, 9424–9433.
Prior to Clemson
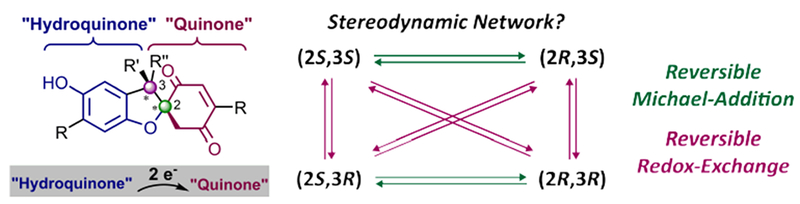
9. A Stereodynamic Redox-Interconversion Network of Vicinal Tertiary and Quaternary Carbon Stereocenters in Hydroquinone-Quinone Hybrid Dihydrobenzofurans
Storch, G.; Kim, B.; Mercado, B. Q.; Miller, S. J.
Angew. Chem. Int. Ed. 2018, 57, 15107–15111.
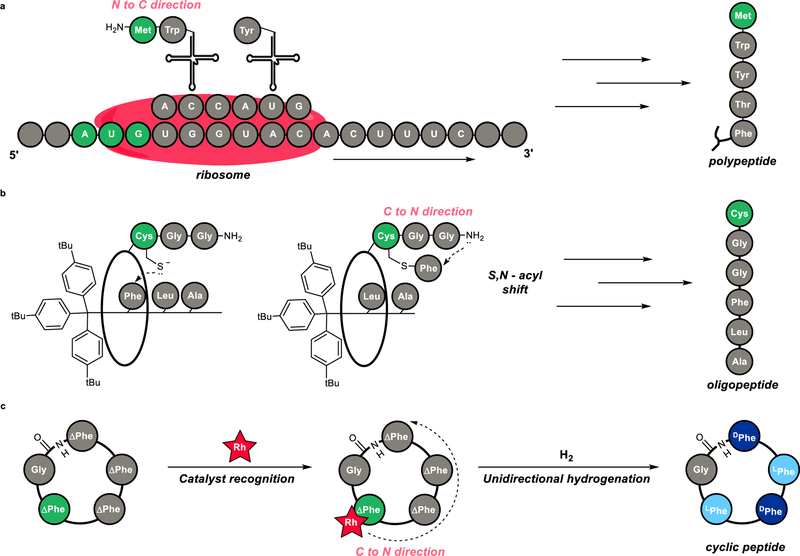
8. Hydrogenation Catalyst Generates Cyclic Peptide Stereocenters in Sequence
Le, D. N.; Hansen, E.; Khan, H. K.; Kim, B.; Wiest, O.; Dong, V. M.
Nat. Chem. 2018, 10, 968–973.
7. Divergent Control of Point and Axial Stereogenicity: Catalytic Enantioselective C−N Bond- Forming Cross-Coupling and Catalyst-Controlled Atroposelective Cyclodehydration
Kwon, Y.; Chinn, A. J.; Kim, B.; Miller, S. J.
Angew. Chem. Int. Ed. 2018, 57, 6251–6255.
6. Enantioselective Intermolecular C–O Bond Formation in the Desymmetrization of Diarylmethines Employing a Guanidinylated Peptide-Based Catalyst
Chinn, A. J.; Kim, B.; Kwon. Y.; Miller, S. J.
J. Am. Chem. Soc. 2017, 139, 18107–18114.
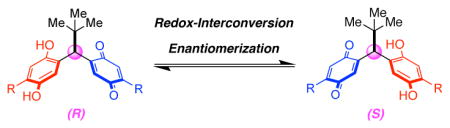
5. Stereodynamic Quinone–Hydroquinone Molecules That Enantiomerize at sp3-Carbon via Redox-Interconversion
Kim, B.; Storch, G.; Banerjee, G.; Mercado, B. Q.; Castillo-Lora, J.; Brudvig, G. W.; Mayer, J. M.; Miller, S. J.
J. Am. Chem. Soc. 2017, 139, 15239–15244.
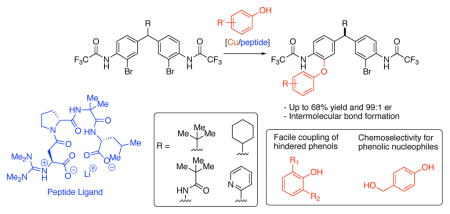
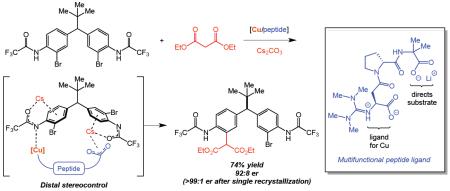
4. Distal Stereocontrol Using Guanidinylated Peptides as Multifunctional Ligands: Desymmetrization of Diarylmethanes via Ullmann Cross-Coupling
Kim, B.; Chinn, A. J.; Fandrick, D. R.; Senanayake, C. H.; Singer, R. A.; Miller, S. J.
J. Am. Chem. Soc. 2016, 138, 7939–7945.
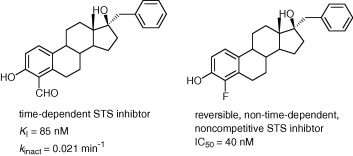
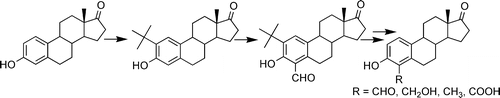
3. Inhibition of Steroid Sulfatase with 4-Substituted Estrone and Estradiol Derivatives
Phan, C.-M.; Liu, Y.; Kim, B.; Mostafa, Y.; Taylor, S. D.
Bioorg. Med. Chem. 2011, 19, 5999–6005.
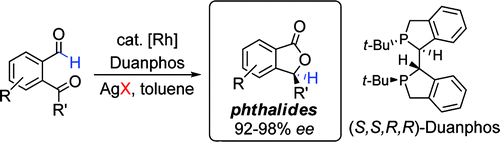
2. Phthalides by Rhodium-Catalyzed Ketone Hydroacylation
Phan, D. H. T.; Kim, B.; Dong, V. M.
J. Am. Soc. Chem. 2009, 131, 15608–15609.
1. Synthesis of 4-Formyl Estrone Using a Positional Protecting Group and Its Conversion to Other C-4-Substituted Estrogens
Liu, Y.; Kim, B.; Taylor, S. D.
J. Org. Chem. 2007, 72, 8824–8830.
Address
Clemson University
Department of Chemistry
Hunter Hall, 211 S. Palmetto Blvd
Clemson, SC 29634, USA